Cleanroom Environmental Monitoring
Your Definitive, Modular, and Turn-key Solutions for Cleanroom Environmental Monitoring
Pharmaceutical Environmental Monitoring Systems to monitor your viable and non-viable particles according to the most recent regulatory requirements. Count, report, document and manage your environmental monitoring data meeting 21 CFR Part 11 data integrity requirements.
Unique modular design allows for quick, easy & scalable installation
The robust and flexible system architecture from Particle Measuring Systems (PMS) ensures cleanroom environmental monitoring data is protected and available whenever there is a need for product release and reporting. Solutions for all cleanroom sizes and classes to fit most budgets.
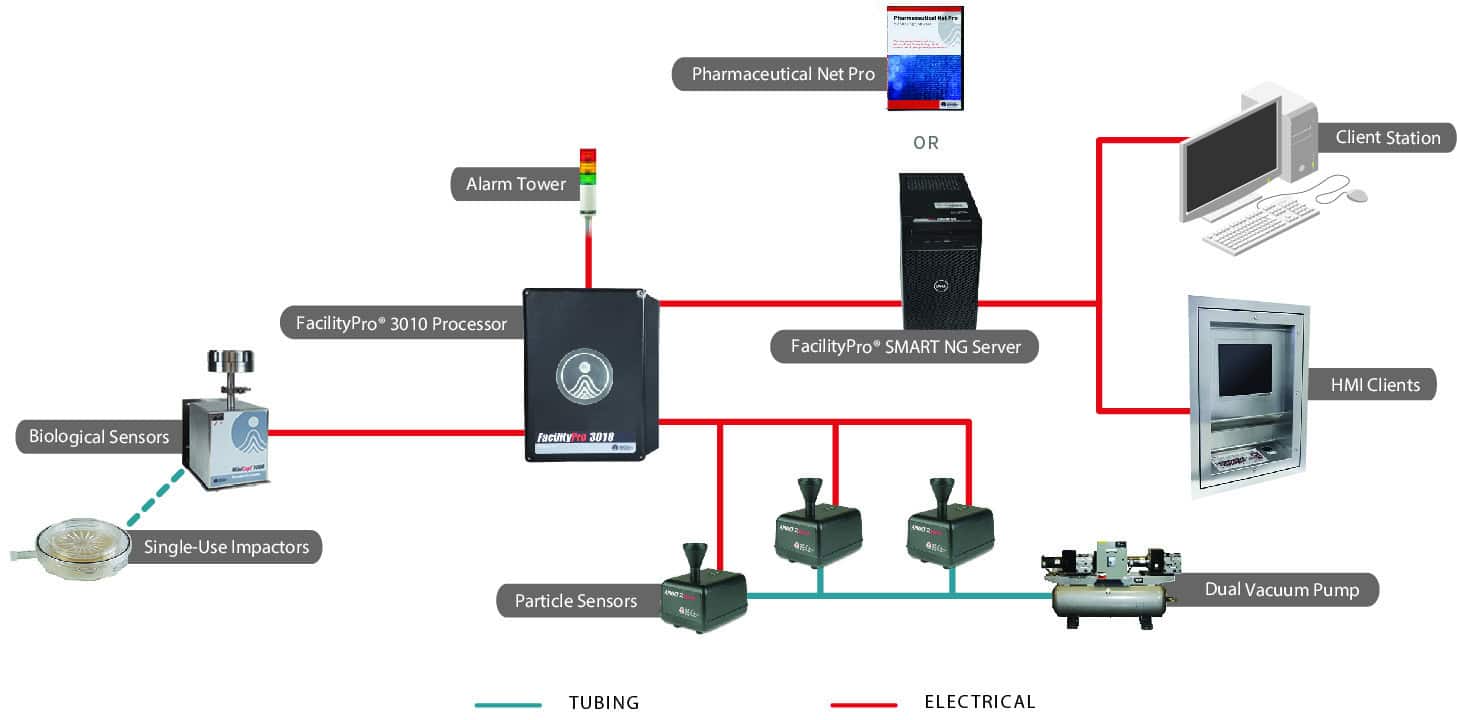
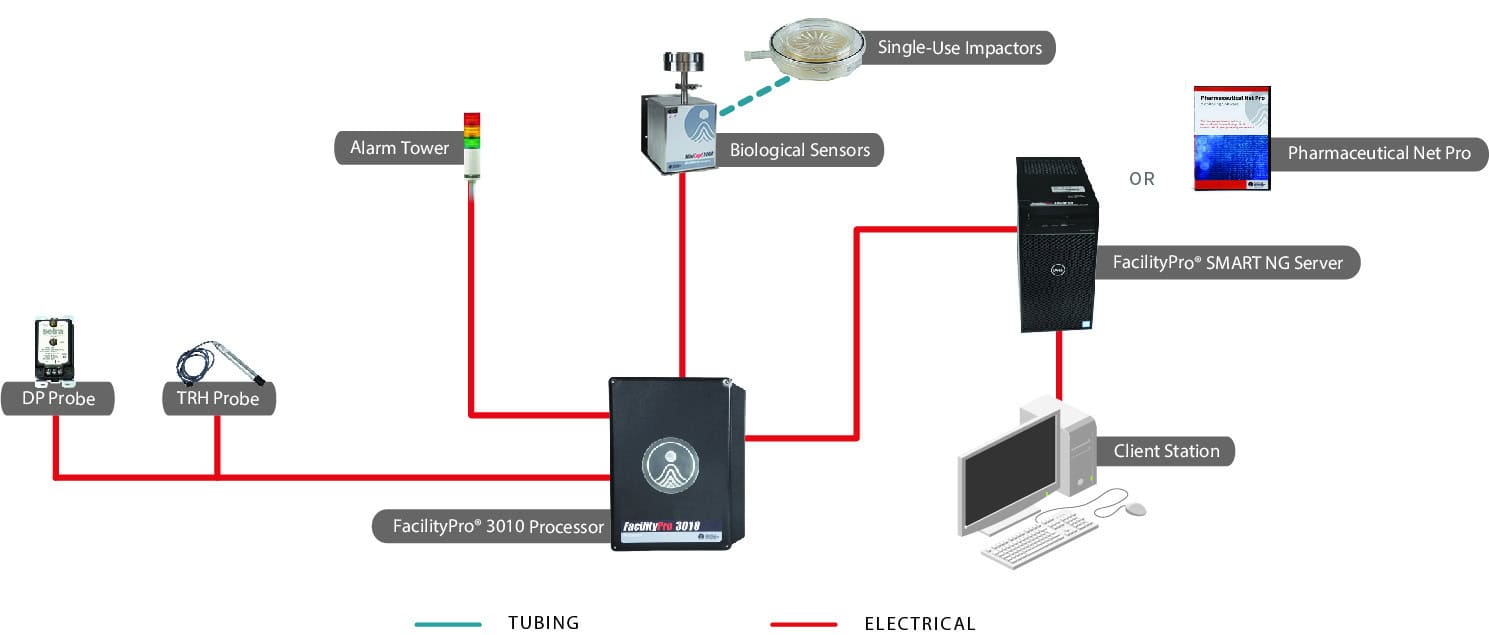
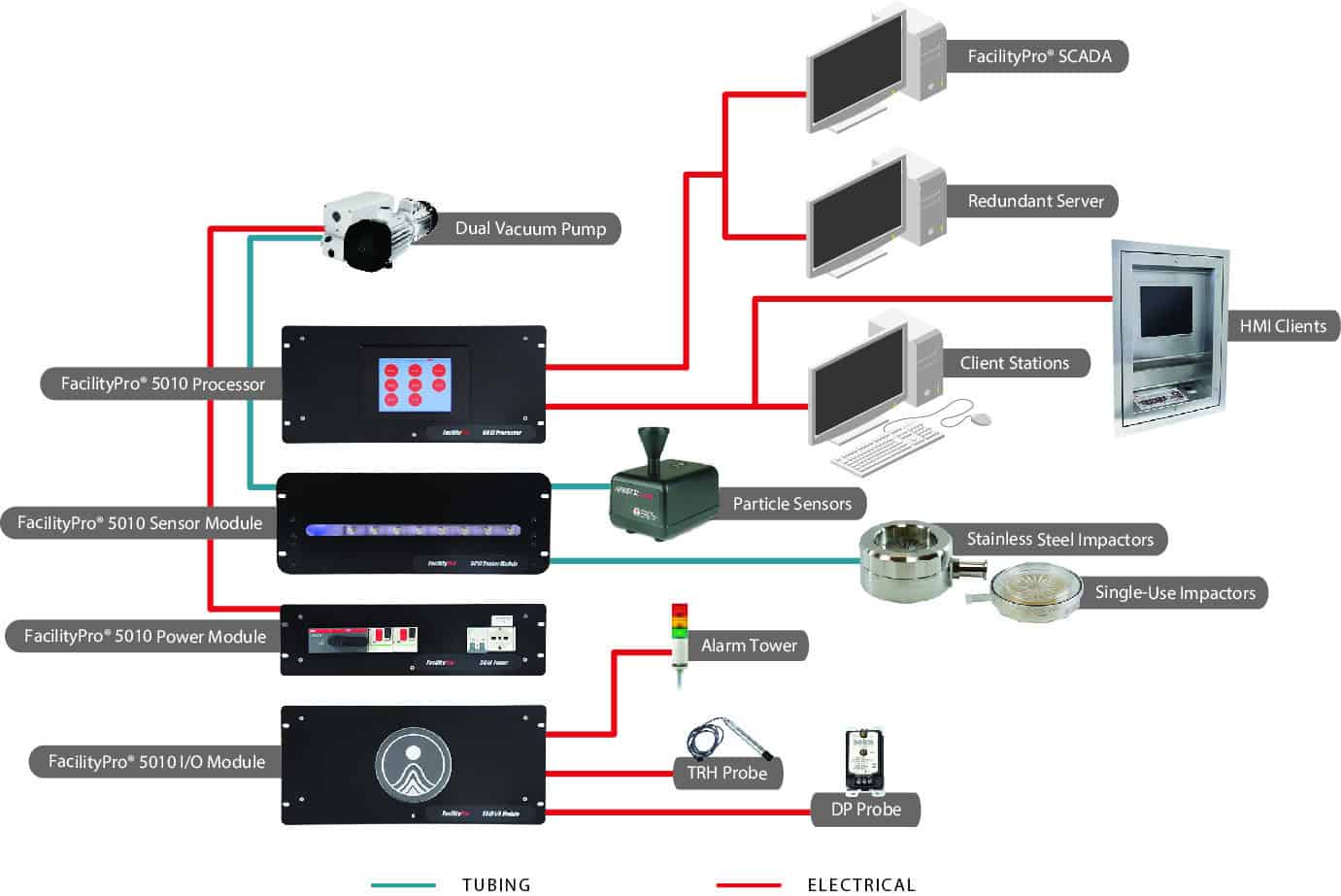
System Configuration Examples
Our modular Systems can be configured to meet your specific needs! Here are three examples, or get a more complete listing of configuration examples.
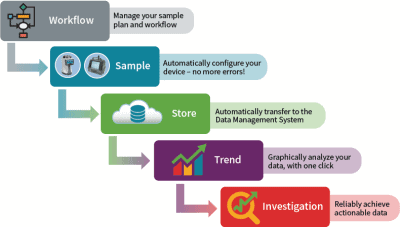
System Automation
Easily collect, report and analyze cleanroom environmental monitoring data. Request a QuoteDetection – Sensors
Viable and non viable samplers from a single manufacturer. Wide variety of analog sensors available including TRH, DAP, Air Flow velocity, etc. Digital inputs and outputs to interface with a selection of operational processes.
Viable Active Air Samplers
Non-Viable Particle Counters
Data and Process Management Software
Enhance your system integration with FacilityPro® SQL Data Exporter and OPC UA drivers to enable plug and play communication with isolator, filling line, LIMS, MES, and other data management software to support accurate decision making and compliance while assuring quick and accurate batch release.
 Request a Quote
Request a Quote
Environmental Monitoring Systems
An environmental monitoring system (EMS) is used in pharmaceutical manufacturing to verify control of the aseptic processing environment and meet regulatory requirements. Particle Measuring Systems offers a variety of EMS solutions to meet different process needs.
Facility Monitoring Software
Particle Measuring Systems is the global leader in contamination monitoring solutions, providing a complete solution consisting of sensors, control systems, and data management software. Particle Measuring Systems’ solutions cover the entire lifecycle of a contamination monitoring project, from initial planning to ongoing support and training.