Quality by Design and Single-Use Air Sampling Approaches
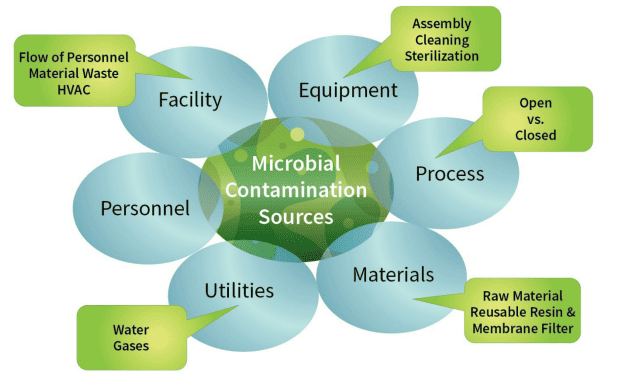 In the 21st century, it is no longer possible to release products or monitor processes (especially aseptically-filled sterile products) using microbiological methodologies and techniques developed in the 20th century. The use of outdated microbiological analytical methods allows the detection of 1/3 of the microorganisms present in the product/process. Therefore, it is not possible to completely identify areas of contamination in the production process.
In the 21st century, it is no longer possible to release products or monitor processes (especially aseptically-filled sterile products) using microbiological methodologies and techniques developed in the 20th century. The use of outdated microbiological analytical methods allows the detection of 1/3 of the microorganisms present in the product/process. Therefore, it is not possible to completely identify areas of contamination in the production process.
Strategies and implementation of more sensitive and/or more reliable microbiological analytical methodologies that allow the identification of potential production process problems and their resolution should be a priority.