Cleanroom Compliance and Classification Standards—Get Your Free Quick-Reference Cards
Fill in your info on the right to get your free Cleanroom Compliance & Classification At-a-Glance cards today!
In pharmaceutical manufacturing, cleanroom compliance is non-negotiable. Navigating the ever-evolving landscape of global cleanroom standards—from the EU GMP Annex 1 to ISO 14644-1 and beyond—can be a daunting task. Whether you’re responsible for cleanroom qualification, contamination control, or regulatory compliance, having quick and accurate access to the right information is crucial.
To make your job easier, the experts at Particle Measuring Systems have compiled the Cleanroom Standards Cards, a set of handy reference guides that distill critical cleanroom requirements from major regulatory bodies into a concise, easy-to-use format.
Why You Need These Cleanroom Standards Cards
Cleanroom classification, monitoring, and control require strict adherence to industry regulations. However, keeping
up with evolving guidelines across multiple governing bodies, such as the FDA, EU, and ISO, can be time-consuming.
Our Cleanroom Compliance Cards serve as a quick-reference tool, allowing you to:
- Quickly compare cleanroom classifications across different standards
- Understand microbial and particle contamination limits at a glance
- Ensure compliance with the latest revisions of global regulatory guidelines
- Improve efficiency in contamination monitoring and cleanroom qualification processes
What’s Inside?
These 2 full-page cards provide at-a-glance summaries of critical cleanroom requirements, including:
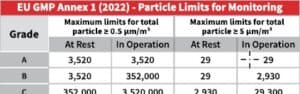
- Cleanroom Classification Levels – Compare particle limits across ISO, EU GMP, and WHO classifications
- Microbial Contamination Limits – Understand the acceptable CFU levels for different cleanroom grades
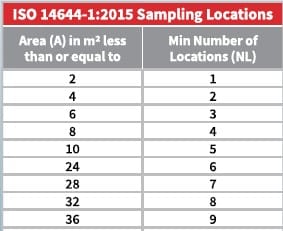
- Regulatory Guidelines Breakdown – Clear insights into key standards from ISO 14644-1, EU GMP Annex 1, and more
This essential reference tool is designed for:
- Quality & Compliance Teams – Ensure adherence to global regulations
- Cleanroom Operators & Engineers – Maintain contamination control best practices
- Regulatory Affairs Professionals – Stay informed on evolving guidelines
Stay Ahead of Regulatory Changes
With increasing scrutiny on pharmaceutical manufacturing, staying compliant is more challenging than ever. The latest updates to EU GMP Annex 1 emphasize a risk-based approach to contamination control, while ISO 14644-1 provides stringent particle count limits. Having a concise, reliable reference can help you stay compliant and audit-ready.
Instead of searching through lengthy regulatory documents, get the information you need in one place.
Get Your Free Copy
Access to accurate, up-to-date regulatory information is key to maintaining cleanroom compliance and ensuring product safety. Download the Cleanroom Compliance Quick-Reference Cards now and simplify your contamination control processes.
By downloading, you’ll gain access to a powerful tool that will help you save time, reduce risk, and ensure compliance in your cleanroom operations. Don’t miss out—get your copy today!
Download your free copy by filling in the form on the right