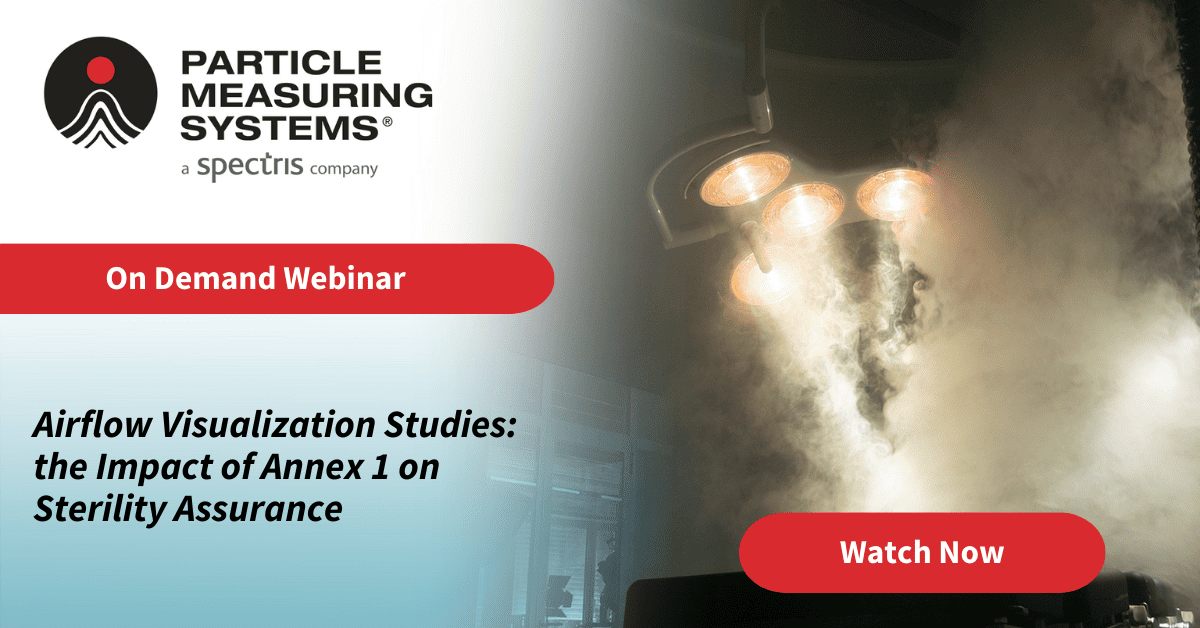
With Annex 1 requiring a risk assessment to determine where sample points should be located, consideration as to those locations and what they mean, in terms of environmental control and monitoring, should be understood.
This webinar will look at the factors that go into a risk assessment to determine location and choice of suitable monitoring equipment and examples of installed sensors in critical locations. Added to this assessment is a secondary function of instrument location vs. sample probe location and that these will need to be connected via tubing, so a review of system design and layout is also to be considered, and understood, if we are to gain the maximum value from the data.
- How to conduct a risk assessment to determine optimal sample point locations in compliance with Annex 1, considering environmental control and monitoring.
- The factors influencing the selection of appropriate monitoring equipment and the practical application of installed sensors in critical areas.
- The relationship between instrument and sample probe placement, including system design and tubing layout, to ensure accurate data collection and analysis.
- Quality Assurance
- Quality Control
- Validation Experts
- Production Teams
- Environmental Monitoring Teams
- Compliance Specialists
- Facility Managers
- Operation Managers
Can’t attend the live event?
Don’t worry! Sign up for one of the sessions and we’ll send you the recording afterwards.