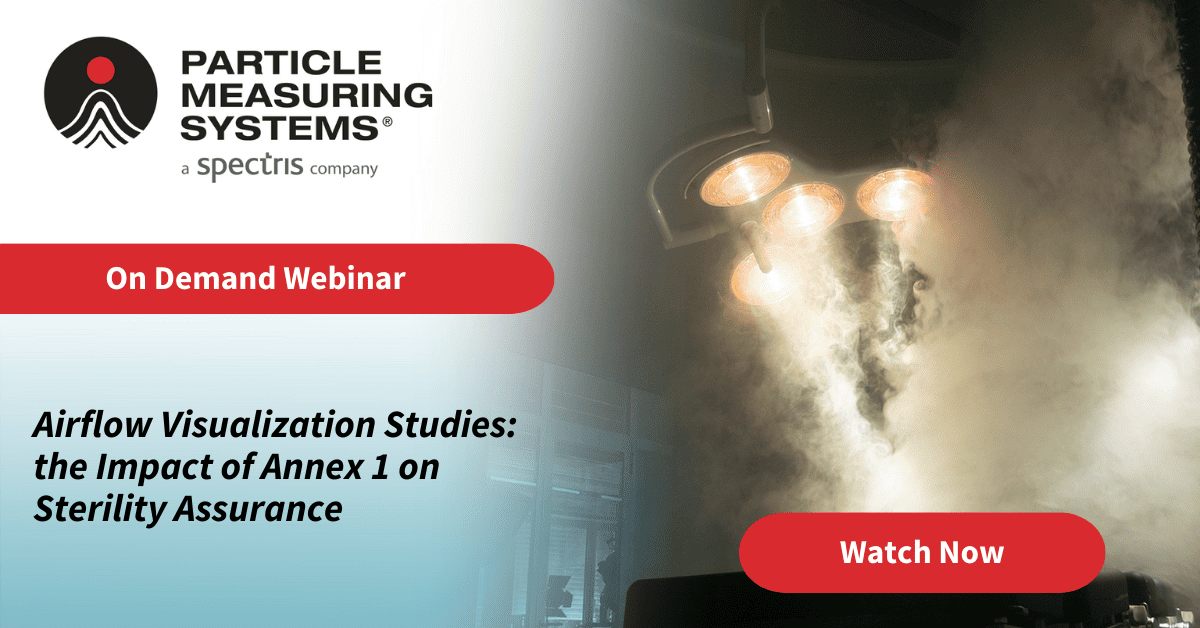
With the recent implementation of the revised Annex 1, airflow visualization studies, such as Smoke Studies and Computational Fluid Dynamics (CFD), have become indispensable tools in a company’s Contamination Control Strategy (CCS).
These studies are no longer confined to the qualification of cleanrooms and clean air equipment. When conducted rigorously, they offer valuable insights for a range of applications, including:
- Environmental Monitoring Plan Development: Optimizing sampling locations for accurate risk assessment.
- Personnel Training: Enhancing understanding of airflow patterns and their impact on contamination control.
- Validation of VHP Cycles: Precisely positioning biological and chemical indicators for reliable cycle validation.
But what constitutes a “properly executed study”? To meet regulatory standards and generate scientifically sound data, several key requirements must be met.
- The Role of Smoke Studies in Contamination Control: Understanding their significance within a comprehensive CCS.
- Documentation Best Practices: Ensuring clear and accurate records of airflow visualization studies.
- Key Requirements for Rigorous Studies: Adhering to industry standards and regulatory guidelines.
- Optimizing Study Frequency: Determining the optimal schedule for effective monitoring.
By the end of this webinar, you’ll gain a deeper understanding of how to leverage airflow visualization studies to build a robust and effective contamination control strategy.
- Quality Assurance
- Quality Control
- Compliance and Validation Specialists
- Bioprocess Engineers
- Regulatory Affairs Specialists
- Manufacturing and Operations Managers
- R&D Personnel
- Pharmaceutical Researchers, Scientists and Consultants