Reducing False Positives in Cleanroom Microbial Air Sampling
In controlled environments like cleanrooms, maintaining sterility is crucial. One of the most important processes in this context is microbial air sampling—a method used to detect airborne contaminants that could jeopardize sterile manufacturing processes. However, one significant challenge in microbial air sampling is the risk of false positives in cleanrooms. These false positives can lead to unnecessary investigations, downtime, and potential product loss, making them a costly issue for industries such as pharmaceuticals, biotechnology, and healthcare.
In this blog, we’ll explore how modern innovations, like the BioCapt® Single-Use microbial impactor, can enhance microbial air sampling efficiency while reducing the risk of false positives in cleanrooms.
 The Role of Microbial Air Sampling in Cleanrooms
The Role of Microbial Air Sampling in Cleanrooms
Microbial air sampling plays a critical role in ensuring cleanroom environments remain uncontaminated. This process involves capturing airborne microorganisms on agar plates or within specialized devices. After sampling, the collected microorganisms are incubated, and any microbial growth is analyzed. The goal is to ensure that cleanroom air is free from contaminants that could compromise the sterility of products or manufacturing processes.
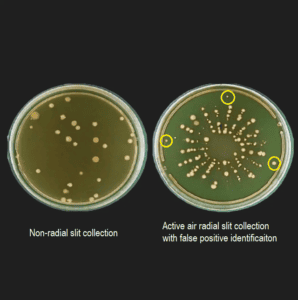
In traditional methods, microbial air sampling often involves stainless steel impactors, which require manual handling of agar plates. During this process, there is a risk of contaminating the plates, leading to false positives—instances where the sample shows contamination that isn’t actually present in the environment. These false positives can create unnecessary concern and additional workload as they trigger investigations into contamination that doesn’t exist.
False Positives in Cleanrooms: A Costly Problem
False positives in cleanrooms are problematic because they result in unneeded interventions and costly delays. In environments where even the slightest contamination can have a dramatic impact—such as pharmaceutical manufacturing or critical healthcare environments—false positives can lead to halted production, expensive investigations, and significant financial losses.
In many cases, these false positives are caused by operator error or contamination during manual handling of sampling equipment, such as when operators touch or manipulate the agar plates during the microbial air sampling process. For industries that rely on sterile conditions, minimizing human intervention and the potential for contamination is key to ensuring accuracy in microbial sampling results.
 How BioCapt Single-Use Microbial Impactor Reduces False Positives
How BioCapt Single-Use Microbial Impactor Reduces False Positives
The BioCapt Single-Use microbial impactor addresses many of the challenges that lead to false positives in microbial air sampling. This innovative device is specifically designed to reduce human intervention, which in turn reduces the chances of contamination during sampling.
Key features of the BioCapt Single-Use microbial impactor include:
- Pre-loaded Sterile Agar Plates: Unlike traditional stainless-steel impactors, which require operators to manually place and handle agar plates, the BioCapt Single-Use comes with pre-loaded sterile agar plates. This eliminates the need for manual intervention, drastically reducing the risk of contamination from human error.
- Sterile, Gamma-Irradiated Design: The entire device is sterile and triple-bagged, undergoing gamma irradiation before use. This ensures that the microbial impactor remains free from contaminants throughout the sampling process.
- Single-Use Convenience: After use, the BioCapt impactor can be discarded, removing the need for time-consuming cleaning or sterilization of reusable equipment. This also eliminates the risk of contamination from improperly cleaned equipment.
- Reduced Operator Handling: By integrating the agar plate into the impactor itself, the BioCapt Single-Use reduces the need for operator intervention. This minimizes touchpoints where contamination could occur, helping to lower the occurrence of false positives.
Improved Sampling Efficiency and Compliance
In addition to reducing false positives, the BioCapt Single-Use microbial impactor offers high sampling efficiency, ensuring that microbial air sampling remains effective in detecting potential contaminants. The impactor’s slit design allows for precise sampling of airborne microorganisms, ensuring that both physical and biological collection efficiencies are maximized.
The BioCapt Single-Use microbial impactor complies with industry standards, including ISO 14698-1, which outlines the principles and methods for controlling biocontamination in cleanroom environments. By meeting these rigorous standards, the BioCapt impactor ensures that sampling data is both reliable and actionable, further enhancing cleanroom contamination control efforts.
Conclusion
For industries where sterility is non-negotiable, such as pharmaceuticals and aseptic manufacturing, the risks posed by false positives in microbial air sampling are significant. These errors not only lead to unnecessary investigations and production downtime but also threaten the integrity of sensitive manufacturing processes.
Innovative solutions like the BioCapt Single-Use microbial impactor offer a way forward by reducing the potential for operator-induced contamination, improving sampling efficiency, and ensuring compliance with industry standards. By adopting such technologies, cleanroom operators can enhance their microbial air sampling processes and reduce the occurrence of costly false positives, ultimately ensuring a safer, more efficient, and contamination-free environment.
TLDR; Key Takeaways:
- Microbial air sampling is essential for detecting airborne contaminants in sterile environments like cleanrooms.
- False positives in cleanrooms can be caused by operator handling errors during sampling, leading to unnecessary costs and delays.
- The BioCapt® Single-Use microbial impactor reduces false positives by eliminating manual handling of agar plates and using a sterile, pre-loaded design.
- By improving sampling efficiency and reducing contamination risk, the BioCapt® Single-Use helps ensure compliance with cleanroom standards, including ISO 14698-1.
- By focusing on modern advancements in microbial air sampling, cleanroom operators can significantly reduce the risk of false positives, ensuring a more reliable and efficient contamination control process.
Want to learn more?