
USP 797 Updates
The most recent revision to USP 797 brings forth changes that touch various aspects of pharmaceutical practices. Here, we explore the USP Regulatory Standards Development Process.

The most recent revision to USP 797 brings forth changes that touch various aspects of pharmaceutical practices. Here, we explore the USP Regulatory Standards Development Process.

This blog post discusses navigating choices in cleanroom viable air monitoring, offering fundamental insights to guide your approach.
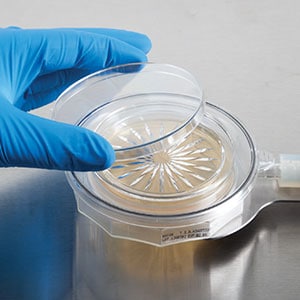
Here we will briefly explore the history, applications, and recent advancements in single-use impactors for active air microbial sampling, with a focus on a groundbreaking single-use alternative.
In 2017, the Food and Drug Administration (FDA) took significant steps towards regulating stem cell therapy, marking a pivotal moment in the industry. This blog provides a background in ATMP sterility testing and the FDA in relation to the paper linked below that outlines guidelines on good manufacturing practice specific to Advanced Therapy Medicinal Products.