Over the last decade, significant technological advances have been made regarding the sterility and quality of new drug and microbiological products. Innovations such as air samplers for microbiological monitoring have played a crucial role in this progress by ensuring accurate detection and control of airborne contaminants. Single Use Devices have streamlined processes and reduced contamination risks, while Rapid Microbiological Tests provide faster, more accurate results, enhancing safety and efficiency. These advancements have led to new treatments and improvements in medical devices, contributing to increased quality of life and life expectancy for millions of patients.
I n 2002, the FDA released its first progress report regarding the regulation of drug product quality. Many updated programs and regulatory documents (most notably, FDA guidance [1, updated 2015]) recommend “building quality into products” by adopting new technological advances, including the use of tools such as air samplers for microbiological monitoring..
n 2002, the FDA released its first progress report regarding the regulation of drug product quality. Many updated programs and regulatory documents (most notably, FDA guidance [1, updated 2015]) recommend “building quality into products” by adopting new technological advances, including the use of tools such as air samplers for microbiological monitoring..
In late 2015 the FDA released a new program allowing pharmaceutical companies to pre-submit questions and proposals regarding the use of innovative technology. The Center for Drug Evaluation and Research (CDER) serves as the primary contact within FDA for companies interested in implementing new testing, processes, or proposed technology while offering direct support.
The technological advances can be segregated into these categories:
- Sterilization and Disinfection – This is a critical process that has been revamped with the use of new technologies such as vaporized hydrogen peroxide, gamma, x-ray, and electron beam irradiation.
- Automation and Robotics – Automation is becoming more prevalent as the advantages are incredible. These advantages include efficiency, increased employee safety, reducing training overhead, eliminating human error, and increasing repeatability. Robotics play a large part in automating many of the aforementioned processes.
- Single Use Technology – This technology eliminates the cleaning and sterilization process allowing for rapid turnover from one product to another or from one bath to another batch. Singe Use systems also decrease overall operating costs by minimizing or reducing CIP/SIP.
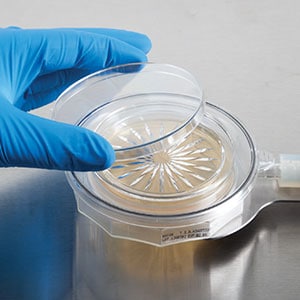
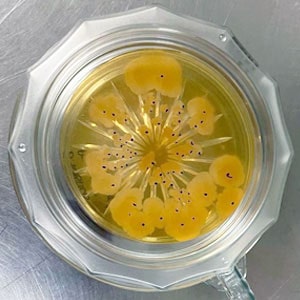
Environmental Monitoring (EM), particularly in Pharmaceutical manufacturing facilities, where the risk of microbial contamination is controlled through aseptic processing, comprises both physical and microbiological test methods. Air samplers for microbiological monitoring play a significant role in this process by providing accurate surveillance of airborne contaminants. Nonviable particulate and viable microbiological surveillance are used to evaluate the design and control of a cGMP-manufacturing environment. The nonviable particulate monitoring program plays an important role as it is used on a routine basis to verify the maintenance of air classifications.
It would be good to have instrumentation capable to detecting more diverse types of microbial contaminants (including damaged, stressed, dormant, and VBNC cells) that usually do not grow on the media described in the guidelines but could potentially grow as an opportunistic infection under special conditions found in clinical patients.
The pharmaceutical industry has been called upon to continually raise its sterility assurance standards through ongoing increased global regulatory standards, especially over the last ten years. Various regulatory agencies expect faster and more complete microbial contamination control. In response to this expectation, suppliers have found a variety of approaches and solutions to meet these needs in a way that both satisfies regulatory requirements and helps pharmaceuticals save time and money by efficiently and accurately providing effective air and surface microbial monitoring. Top solutions for pharmaceutical contamination control meet at least one of the following criteria: sterility requirements, automation, and single use.
Learn more by downloading this paper.