According to USP 797 guidelines, critical areas must continuously meet ISO Class 5 or better conditions for 0.5 μm particles and must exclude microbial contamination during the compounding of CSP’s. These classifications ensure that a designated zone is maintained in a clean state. The monitoring program is designed based on contamination risk to finished product quality.
According to USP 797 guidelines, critical areas must continuously meet ISO Class 5 or better conditions for 0.5 μm particles and must exclude microbial contamination during the compounding of CSP’s. These classifications ensure that a designated zone is maintained in a clean state. The monitoring program is designed based on contamination risk to finished product quality.
An effective USP 797 environmental monitoring program provides meaningful information on the quality of the compounding environment and any environmental trends in surrounding areas.
In addition, an effective environmental monitoring program will identify potential routes of contamination, allowing for the implementation of corrections to prevent CSP contamination.
Particle Monitoring to Meet USP 797 Guidelines (Non-Viable)
The Parenteral Drug Association (PDA) issued a recommendation for routine monitoring for all Aseptic Grade areas using portable particle counting devices. The recommendations are as follows:
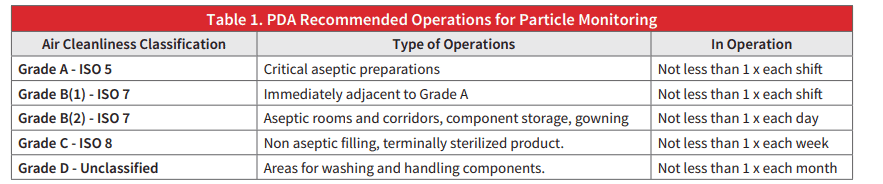
Rooms are classified according to function.
- Ante-area (Transition areas) – Must meet at least ISO Class 8 standards.
- Buffer area – Must meet at least ISO Class 7 standards.
- CSP preparation areas – Must meet at least ISO Class 5 standards.
ISO standards also limit the amount of particulates that can be in each class of area
The paper includes information on the most recent 797 Guideline updates and recommendations.
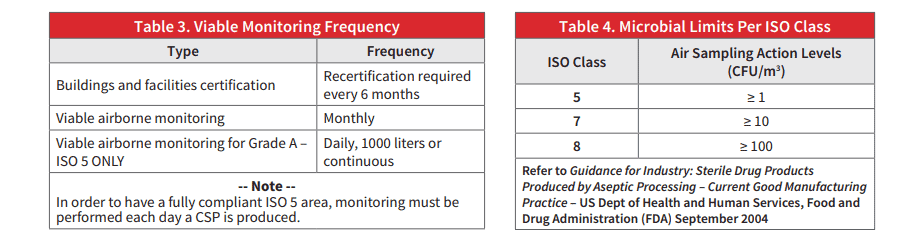
Complete the form to download the full paper…
Particle Measuring Systems can help you meet your USP 797 monitoring requirements with these solutions:
- MiniCapt Mobile Microbial Air Sampler
- BioCapt Single-Use Microbial Impactor for viable monitoring
- Lasair Pro Portable Airborne Particle Counter